Immunotherapy Research Group

The Immunotherapy Research Group is engaged in research and development of immune system-based therapies for cancer treatment. Our research covers a wide range of studies focused on stimulating, modifying or enhancing the anti-cancer immune responses. The Group strives to identify new therapeutic targets and develop innovative technologies that can increase the effectiveness and safety of immunotherapy. Methodologically, we are interested in visualizing the immune cell dynamics in tissues, especially the cancer microenvironment for which we use intravital (confocal and two-photon) microscopy and image analysis tools including artificial intelligence approaches.
The insufficient effectiveness of conventional oncological therapies was the driving force to use the immune system’s potential to fight cancer. Immunotherapy approach employs different strategies of immune cell mobilization for destruction of malignant cells. One of them is adoptive cell therapy (ACT) which relies on infusion of cancer-specific T lymphocytes capable of killing target cells. The progress in cell engineering technology enabled in vitro generation of modified immune cells with improved cytotoxic efficacy. So far, six ACTs based on genetically modified, patient-derived T lymphocytes (so called chimeric antigen receptor/CAR T cells) have been approved for clinical use to treat a limited number of blood-derived cancers. Further improvements to ACT involve identification of better effector cells which do not require an autologous setting. One promising candidate is a subset of T cells expressing a T cell receptor (TCR) with γ and δ chains.
γδ T lymphocytes constitute 1-10% of blood circulating T cells and are a puzzling cells bridging innate and adaptive immune responses. This feature renders them more suitable for immunotherapy than prevailing αβ TCR-bearing T lymphocytes which require antigen processing and subsequent presentation by major histocompatibility complex (MHC) molecules. γδ TCRs are poised to directly recognize molecular patterns of cell pathology (stress antigens) without the need for MHC. Importantly, the lack of MHC restriction enables allogeneic ACT with γδ T cells in situations when the expansion of autologous T cells becomes difficult or impossible. Upon activation, γδ T lymphocytes produce interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α), well-known cytokines with cytotoxic activity. It has been demonstrated that a dominant subset of γδ T cells in blood, with a Vδ2 chain in the TCR complex, responds to phospho-antigens, which are non-peptidic phosphorylated intermediates of the non-mevalonate and mevalonate pathways of isoprenoid biosynthesis. Another major subpopulation of γδ T cells with a Vδ1 chain, confined mostly to skin and mucosa, recognizes molecules expressed on stressed and tumor cells such as MHC class I-related molecules A and B (MICA/B) and UL16-binding proteins. Moreover, both subsets express natural-killer group 2, member D (NKG2D) molecule, which also binds stress antigens, such as MICA/B, and can efficiently trigger the antitumor functions. Many preclinical data obtained in laboratory mice showed the efficacy of blood-derived and in vitro expanded γδ T lymphocytes against various tumors, which enabled their further testing in ongoing early phases of clinical trials.
γδ T cell highlights:

- cytotoxic functions depending on γδ TCR and NKG2D recognition of ”cell pathology antigens”
- unclear identity of γδ TCR ligands (in contrast to NKG2D ligands)
- fast response without antigen presentation (in contrast to αβ T cells)
- insensitivity to MHC molecules (in contrast to NK cells)
Even though some γδ TCR ligands have been identified, still, it is not entirely clear how γδ T cells get activated mechanistically and which molecules apart from γδ TCR are important. Thus, our aim is to investigate γδ TCR signaling and γδ T cell activation mechanisms in general, which can be next translated into creation of more efficient cancer-killing effector cells. This includes also genetic modification of γδ T cells to improve their effector functions. Our ultimate goal is to bring the research outcomes as an IP-protected γδ T cell-based product to the clinic and help cancer patients.
Research pipeline: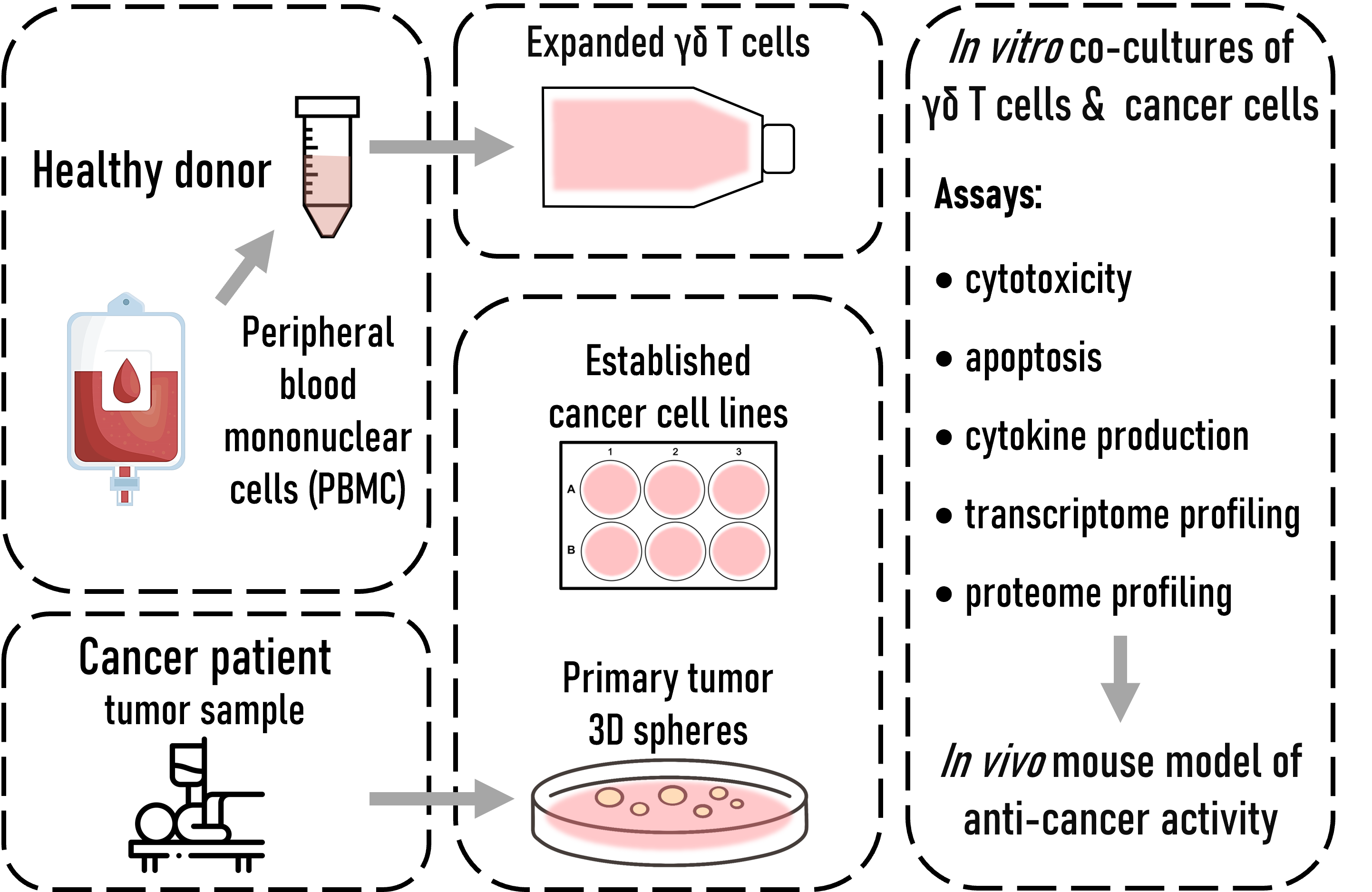
Using in vitro cultures of blood-derived γδ T cells, we assess their cytotoxic functions toward cancer cell lines and patient-derived primary tumor cells to determine which subset (Vδ2+ or Vδ1+) suits best for immunotherapeutic use. We set up three-dimensional tumor cell cultures to examine and compare cancer-killing efficacy of γδ T cells. In the next step, tumor killing by γδ T lymphocytes will be verified in vivo in a mouse model.

Grzegorz Chodaczek, PhD, principal investigator
Grzegorz graduated from the Wroclaw Medical University in 2001, where he studied at the Faculty of Pharmacy. In 2007, he received PhD degree in biology from the Institute of Immunology and Experimental Therapy, PAS in Wroclaw based on his studies on adjuvant properties of lactoferrin for vaccine formulations. Between 2005-2007, he worked as a research associate at the University of Texas Medical Branch in Galveston, USA, in Dr. Istvan Boldogh’s lab, studying reactive oxygen species’ role in allergic reactions. In 2007, he started a postdoctoral fellowship at the University of Texas MD Anderson Cancer Center in Houston, USA, in Dr. Tomasz Zal’s lab. His project involved microscopic visualization of immune surveillance of skin and lung tumor microenvironment. In 2011, he took a joint position of a microscopy core manager at La Jolla Institute for Immunology, San Diego, USA, and an instructor in Dr. Mathias von Herath’s lab, where he conducted imaging-driven research on immune system involvement in several diseases (type 1 diabetes, viral infections, Crohn’s disease, atherosclerosis and cancer). In 2014, he joined Wroclaw Research Centre EIT+ (currently Łukasiewicz-PORT) to help in creation of the state-of-the-art imaging facility. In 2023, he established his own research group focused on immune cell-based therapies of cancer. His goal is develop innovative technologies that can increase the effectiveness of immunotherapy.

Natalia Frankiewicz, BSc, laboratory manager
Natalia completed her engineering studies in Biotechnology at the Wroclaw University of Science and Technology. She gained experience in laboratory work in an analytical laboratory, deepening her knowledge in the field of implementation and quality management. In our group, she works as a lab manager, offering support to group members in their daily tasks. She is responsible for monitoring the lab inventory and coordinating orders. She is also responsible for budget control and documentation. She supports the group in histology and immunohistochemistry techniques. She is involved in collaboration with pathologists, processing human samples and preparing histopathological microscopic slides that are used in diagnostics.

Justyna Mączyńska, PhD, postdoctoral researcher
Justyna graduated in Biotechnology from Wroclaw University of Science and Technology and received her PhD in the Department of Medical Biochemistry at Wroclaw Medical University. During her doctoral studies, she was awarded the Preludium grant and, as part of the Erasmus+ program and Etiuda studentship, gained extensive research experience at the Preclinical Molecular Imaging Team within the Institute of Cancer Research in London, where she also conducted her initial postdoctoral training. Her primary focus revolved around developing anti-cancer photoimmunotherapy, leveraging near-infrared light and small photoactive conjugates to target and treat malignant cells. She investigated mechanisms underlying the transformation of an immunosuppressive tumor microenvironment into an immune-vulnerable one. Currently, as a member of our team, she is dedicated to immunotherapeutic approaches for treating glioblastoma, specifically utilizing γδ T cells as part of an adoptive cell therapy, and aims to gain deeper insights into the mechanisms governing cell interactions within the intricate tumor microenvironment.

Agnieszka Chwastek, PhD, postdoctoral researcher
Agnieszka earned BSc and MSc degrees (in Biology and Neurobiology, respectively) from Jagiellonian University in Cracow, and received her PhD in Medical Biology from the Maj Institute of Pharmacology PAS in Cracow. Her research focuses on investigating connections within the neuro-immune cellular network, a topic she has previously explored in the context of neurodegenerative diseases and animal models of neuropathic pain. As a post-doctoral researcher in our team, Agnieszka works on gene silencing in primary γδ T lymphocytes and optimization of a humanized mouse model for human glioblastoma studies.

Agnieszka Szyposzyńska, MSc, process engineer
Agnieszka graduated from the Jagiellonian University in Cracow, where she studied Molecular Biotechnology. As a part of her master’s thesis, she determined the impact of extracellular vesicle isolation methods on their proangiogenic potential and she participated in projects related to stem cells and extracellular vesicles biology. After graduation, she started a job at the Institute of Immunology and Experimental Therapies PAS in the project Database of Scientific Information Supporting Innovative Therapies aimed at digitizing and providing information about mesenchymal stem cells on a digital platform. Currently, she is finishing her PhD at the Institute of Immunology and Experimental Therapies PAS. During her PhD, she studied the impact of microvesicles derived from mesenchymal stem cells on the biological activity of ovarian cancer cell lines and primary ovarian cancer cells from post-operative tissue and ascitic fluid. In the Immunotherapy Group, she supports the team in daily laboratory activities and cell culture.
Szyposzynska A, Bielawska-Pohl A, Murawski M, Sozanski R, Chodaczek G, Klimczak A. Mesenchymal Stem Cell Microvesicles from Adipose Tissue: Unraveling Their Impact on Primary Ovarian Cancer Cells and Their Therapeutic Opportunities. Int. J. Mol. Sci. 2023; 24(21):15862. [link].
Skulska K, Kędzierska AE, Krzyżowska M, Chodaczek G. Age-Related Changes in Female Murine Reproductive Mucosa with respect to γδ T Cell Presence. J Immunol Res. 2023: 3072573. [link].
Mączyńska J, Raes F, Da Pieve C, Turnock S, Boult JKR, Hoebart J, Niedbala M, Robinson SP, Harrington KJ, Kaspera W, Kramer-Marek G. Triggering anti-GBM immune response with EGFR-mediated photoimmunotherapy. BMC Med. 2022;20(1):16. [link].
Kupczyk P, Simiczyjew A, Marczuk J, Dratkiewicz E, Beberok A, Rok J, Pieniazek M, Biecek P, Nevozhay D, Slowikowski B, Chodaczek G, Wrzesniok D, Nowak D, Donizy P. PARP1 as a Marker of an Aggressive Clinical Phenotype in Cutaneous Melanoma-A Clinical and an In Vitro Study. Cells 2021; 10(2):286. [link].
Chodaczek G, Pagni PP, Christoffersson G, Ratliff SS, Toporkiewicz M, Wegrzyn AS, von Herrath M. The effect of Toll-like receptor stimulation on the motility of regulatory T cells. J Autoimmun. 2021; 116:102563.[link].
Kulbacka J, Chodaczek G, Rossowska J, Szewczyk A, Saczko J, Bazylińska U. Investigating the photodynamic efficacy of chlorin e6 by millisecond pulses in metastatic melanoma cells. Bioelectrochemistry. 2020; 138:107728. [link].
Mączyńska J, Da Pieve C, Burley TA, Raes F, Shah A, Saczko J, Harrington KJ, Kramer-Marek G. Immunomodulatory activity of IR700-labelled affibody targeting HER2. Cell Death Dis. 2020; 11(10):886. [link].
Szyposzynska A, Bielawska-Pohl A, Krawczenko A, Doszyn O, Paprocka M, Klimczak A. Suppression of Ovarian Cancer Cell Growth by AT-MSC Microvesicles. Int J Mol Sci. 2020; 21(23):9143. [link].
Skulska K, Wegrzyn AS, Chelmonska-Soyta A, Chodaczek G. Impact of tissue enzymatic digestion on analysis of immune cells in mouse reproductive mucosa with a focus on γδ T cells. J Immunol Methods. 2019; 474:112665. [link].
Mikolajewicz K, Chodaczek G. Going deeper: three-dimensional study of γδ T cells in mouse reproductive tract using tissue clearing methods. Immunol Cell Biol. 2019; 97(1):104-111. [link].
Mączyńska J, Choromańska A, Kutkowska J, Kotulska M, Zalewski M, Zalewski J, Kulbacka J, Saczko J. Effect of electrochemotherapy with betulinic acid or cisplatin on regulation of heat shock proteins in metastatic human carcinoma cells in vitro. Oncol Rep. 2019; 41(6):3444-3454. [link].
Jurga AM, Rojewska E, Makuch W, Mika J. Lipopolysaccharide from Rhodobacter sphaeroides (TLR4 antagonist) attenuates hypersensitivity and modulates nociceptive factors. Pharm Biol. 2018; 56(1):275-286. [link].
Chodaczek G, Toporkiewicz M, Zal MA, Zal T. Epidermal T Cell Dendrites Serve as Conduits for Bidirectional Trafficking of Granular Cargo. Front. Immunol. 2018; 9:1430. [link].
Orlowski P, Tomaszewska E, Ranoszek-Soliwoda K, Gniadek M, Labedz O, Malewski T, Nowakowska J, Chodaczek G, Celichowski G, Grobelny J, Krzyzowska M. Tannic acid-modified silver and gold nanoparticles as novel stimulators of dendritic cells activation. Front. Immunol. 2018; 9:1115. [link].
Christoffersson G, Chodaczek G, Ratliff SS, Coppieters K, von Herrath MG. Suppression of diabetes by accumulation of non-islet-specific CD8+ effector T cells in pancreatic islets. Sci Immunol. 2018; 3(21): eaam6533. [link].
Borek A, Sokolowska-Wedzina A, Chodaczek G, Otlewski J. Generation of high-affinity, internalizing anti-FGFR2 single-chain variable antibody fragment fused with Fc for targeting gastrointestinal cancers. PLoS One 2018; 13(2):e0192194. [link].
Weżgowiec J, Kulbacka J, Saczko J, Rossowska J, Chodaczek G, Kotulska M. Biological effects in photodynamic treatment combined with electropermeabilization in wild and drug resistant breast cancer cells. Bioelectrochemistry 2018; 123:9-18. [link].
Jurga AM, Piotrowska A, Makuch W, Przewlocka B, Mika J. Blockade of P2X4 Receptors Inhibits Neuropathic Pain-Related Behavior by Preventing MMP-9 Activation and, Consequently, Pronociceptive Interleukin Release in a Rat Model. Front Pharmacol. 2017; 8:48. [link].
Jurga AM, Rojewska E, Piotrowska A, Makuch W, Pilat D, Przewlocka B, Mika J. Blockade of Toll-Like Receptors (TLR2, TLR4) Attenuates Pain and Potentiates Buprenorphine Analgesia in a Rat Neuropathic Pain Model. Neural Plast. 2016; 2016:5238730. [link].
Shaked I, Hanna RN, Shaked H, Chodaczek G, Nowyhed HN, Tweet G, Tacke R, Basat AB, Mikulski Z, Togher S, Miller J, Blatchley A, Salek-Ardakani S, Darvas M, Kaikkonen MU, Thomas GD, Lai-Wing-Sun S, Rezk A, Bar-Or A, Glass CK, Bandukwala H, Hedrick CC. Transcription factor Nr4a1 couples sympathetic and inflammatory cues in CNS-recruited macrophages to limit neuroinflammation. Nat Immunol. 2015; 16(12):1228-34. [link].
Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC. Patrolling monocytes control tumor metastasis to the lung. Science. 2015; 350(6263):985-90. [link].
Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Marée AF, Zal T, de Boer RJ, Haanen JB, Schumacher TN. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci USA. 2012; 109(48):19739-44. [link].
Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012; 122(9):3114-26. [link].
Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol. 2012; 13(3):272-82. [link].
Zal T, Chodaczek G. Intravital imaging of anti-tumor immune response and the tumor microenvironment. Semin Immunopathol. 2010; 32(3):305-17. [link].
Chodaczek G, Bacsi A, Dharajiya N, Sur S, Hazra TK, Boldogh I. Ragweed pollen-mediated IgE-independent release of biogenic amines from mast cells via induction of mitochondrial dysfunction. Mol Immunol. 2009; 46(13):2505-14. [link].
Chodaczek G, Zimecki M, Lukasiewicz J, Lugowski C. A complex of lactoferrin with monophosphoryl lipid A is an efficient adjuvant of the humoral and cellular immune response in mice. Med Microbiol Immunol (Berl). 2006; 195(4):207-16. [link].
Current projects:
- National Science Centre. OPUS, funding period: 2020-2025; budget: 3 374 760 PLN; Analysis of the effectiveness of glioblastoma immunotherapy using gamma-delta T cells. Role: Principal Investigator
- National Science Centre. SONATA BIS, funding period: 2022-2026; budget: 3 985 000 PLN (485 040 PLN to Łukasiewicz-PORT); Spectroscopic and microscopic techniques in nano-probing, modeling and recognition of interactions between erythrocytes and vascular endothelial cells at the molecular level. Role: Consortium Partner
Past projects:
- National Science Centre. SONATA BIS, funding period: 2015-2021; budget: 1 997 904 PLN; Multiparameter characterization of surveillance of reproductive epithelium homeostasis by gamma-delta T cells that includes high resolution intravital microscopy. Role: Principal Investigator
- National Science Centre. OPUS, funding period: 2015-2019, budget: 1 287 623 PLN; Constitutive autoreactivity of gamma-delta T cells as a beneficial mechanism of epidermal barrier surveillance. Role: Principal Investigator
If you are interested in joining our team, contact us at: grzegorz.chodaczek@port.lukasiewicz.gov.pl
We are currently looking for a PhD student for a project devoted to a humanized mouse model of ovarian cancer patient (expected project start at Q2 2024).

