Synaptogenesis Group

PRINCIPAL INVESTIGATOR Tomasz J. Prószyński, Ph.D.
Our Laboratory is aiming in understanding mechanisms that regulate organization of synapses in the central and the peripheral nervous systems. We are committed to perform cutting edge innovative and interdisciplinary research using wide range of molecular, cellular and genetic techniques. Part of our mission to provide an intensive training for scientific and personal development of our team members with the intention to develop new generation of first league scientists and valuable members of the society.
Our publication Rojek et al., PloS Biology (2019) was awarded Konorski Award 2020 for the best publication in neuroscience in Poland in 2019. The Konorski Award is a joint award of the Polish Neuroscience Society and The Committee of Neurobiology of the Polish Academy of Sciences. Congratulations to all authors!
Tomasz J. Prószyński, Ph.D., principal investigator
Tomasz Prószyński performed his Ph.D. training at the Kai Simons’ Laboratory at the Max-Planck Institute for Cell Biology and Genetics in Dresden (Germany). His research has been focused on intracellular protein sorting and mechanisms of cell polarity in yeast as a model system. After Ph.D. Tomasz Prószyński decided to use his training in cell biology in a more complex system, he joined Joshua Sanes’ Laboratory at Harvard University (USA), where he studied how muscle cells orchestrate the postsynaptic machinery at the neuromuscular junction (NMJ). His research has been mostly conducted on cultured muscle cells stimulated to form postsynaptic apparatus. In 2013, Tomasz Prószyński established his laboratory at the Nencki Institute of Experimental Biology where he was leading projects aiming in understanding the molecular mechanisms that regulate the formation and maintenance of the NMJ using a mouse as a model system. In parallel, Tomasz Prószyński and his team conducted projects that focus on the understanding formation of synapses in the brain and the organization of neuronal networks. Since 2020, Tomasz Prószyński is leading Synaptogenesis Laboratory at the Łukasiewicz – PORT.
Team members:
- Bhola Shankar Pradhan, Ph.D.
- Anthony Kischel, Ph.D.
- Przemysław Kaczor, Ph.D.
- Małgorzata Sotomska, Ph.D.
- Yauhen Bandaruk, Ph.D.
- Iana Garmash, Ph.D.
- Olga Wójcicka, Ph.D.
- Przemysław Duda, Ph.D.
- Teresa De Cicco, M.Sc.
- Joanna Sadlak, M.Sc.
- Margareta Jabłońska, M.Sc.
- Ila Joshi, M.Sc.
- Angelymar Medina Moreno
- Monika Klejner, M.Sc.
- Martyna Wołoszka, M.Sc.
- Wioletta Cipper, M.Sc.
Please contact us if you are interested in a position in our group: tomasz.proszynski@port.lukasiewicz.gov.pl
Our laboratory is conducting innovative multidisciplinary research to understand the organization and function of synapses in the central nervous system and those between motor neurons and skeletal muscles. We use mouse models including conditional knockout and transgenic mice as well as cultured cells to decipher molecular machinery that orchestrates synapses in physiology and disease.
Brain research
Our recent work has identified Amot and Yap as critical regulators of dendritic tree morphology in cultured neurons and Purkinje cells in vivo. Conditional knockout mice with depletion of Amot or Yap in neurons had morphological abnormalities in cerebellar origination and impaired locomotor organization. Our studies revealed that Amot and Yap-dependent organization of dendrites involves S6 kinase and phosphorylation of ribosomal protein S6.
Rojek KO, Krzemień J, Doleżyczek H, Boguszewski PM, Kaczmarek L, Konopka W, Rylski M, Jaworski J, Holmgren L, Prószyński TJ. „Amot and Yap1 regulate neuronal dendritic tree complexity and locomotor coordination in mice”. PLoS Biol. 2019 May 1;17(5):e3000253.
This publication has been awarded the Konorski Award for the best publication in neuroscience in Poland in 2019. The Konorski Award is a joint award of the Polish Neuroscience Society (PTBUN) and the Committee of Neuroscience of the Polish Academy of Sciences.
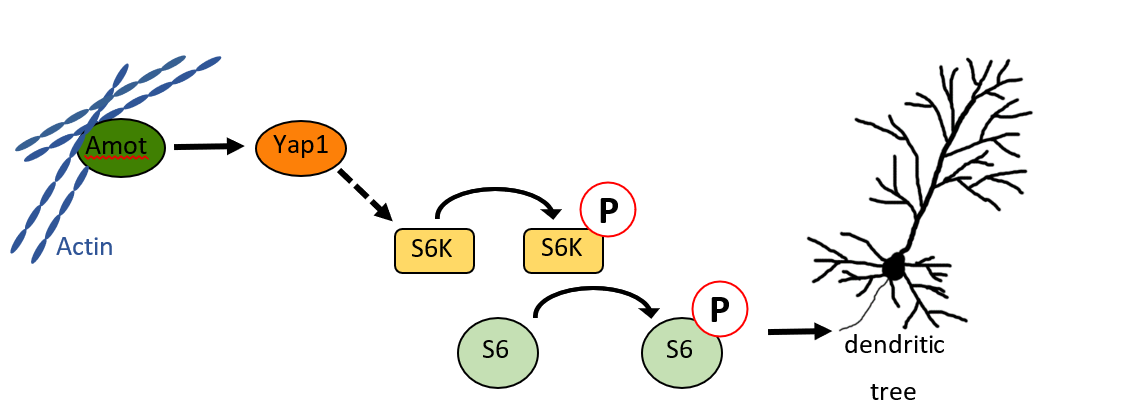
Rojek et al., PLoS Biology 2019
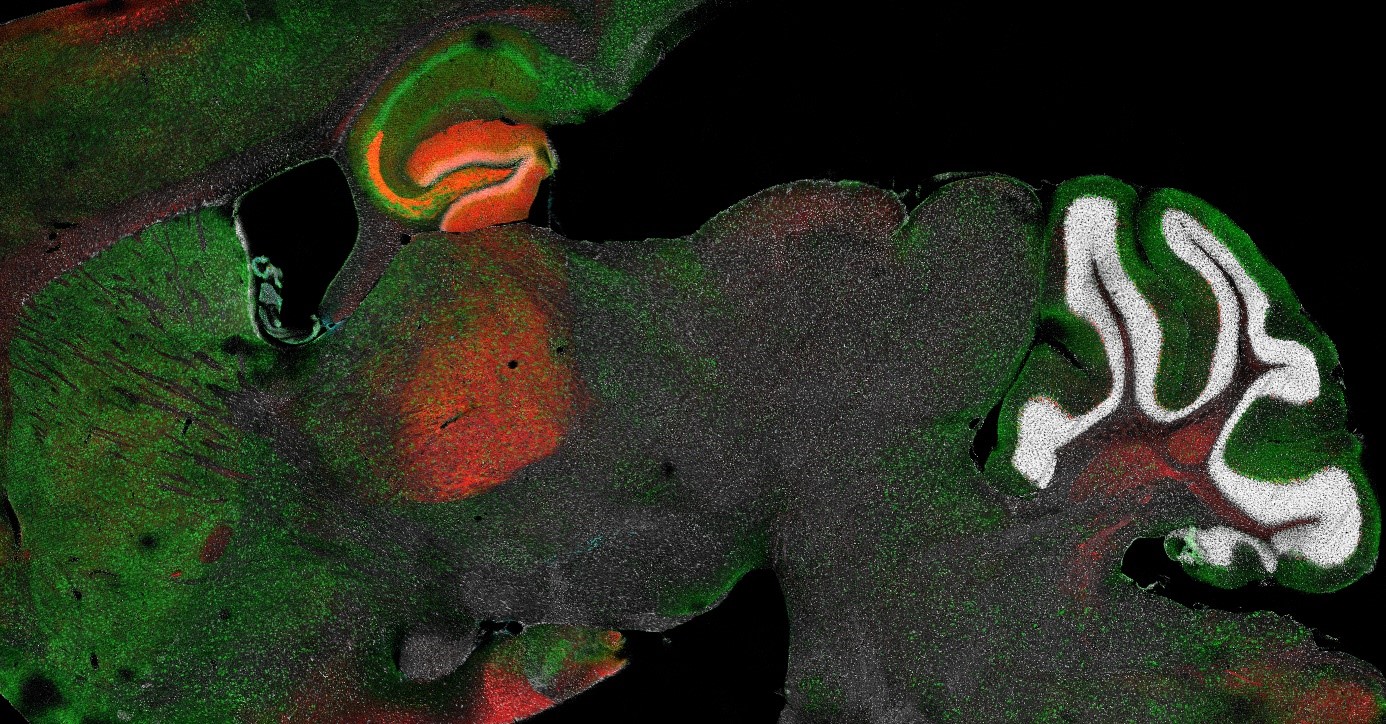
Figure: Brain immunohistochemistry (courtesy of Anthony Kischel).
Neuromuscular research
Neuromuscular junctions (NMJs) are specialized synapses formed between motor neurons and skeletal muscle fibers. These structures are responsible for the transmission of information from the central nervous system to skeletal muscles allowing for their contraction. Proper functioning of NMJs is crucial for our voluntary movements, maintenance of the body posture, and breathing. Therefore, malfunctioning of NMJs leads to serious neuromuscular disorders characterized by muscle wastage, loss of strength, and respiratory problems. It is estimated that there are over 300 neuromuscular disorders and many of them are of unknown etiology. Thus, it is important to understand the mechanisms underlying the organization and functioning of these synapses.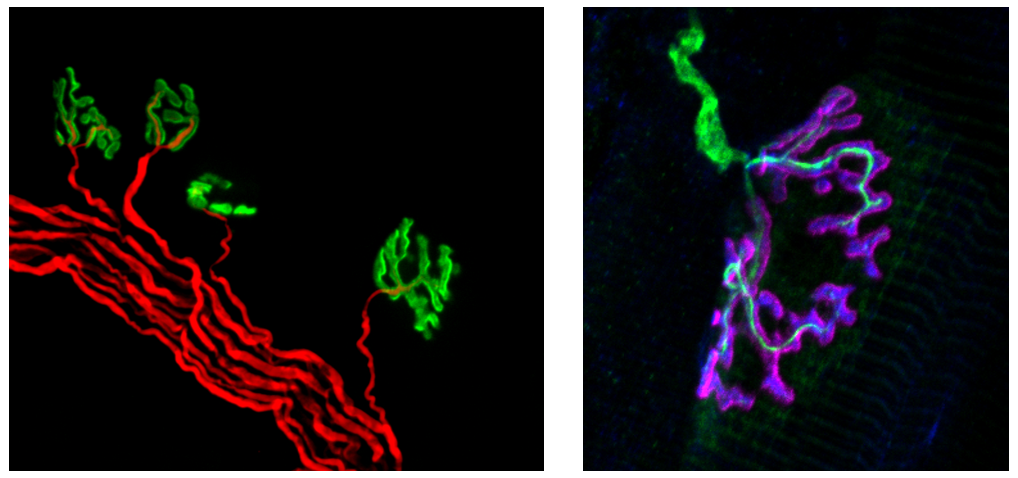
Figure: Motor neurons (red on the right image and green on the left image) innervate postsynaptic machinery (AChR, green on the right, and purple on the left) on the muscle fibers (the muscle contractile machinery is visible as stripes on the right image). Courtesy of Teresa De Cicco.
The Dystroglycan Complex
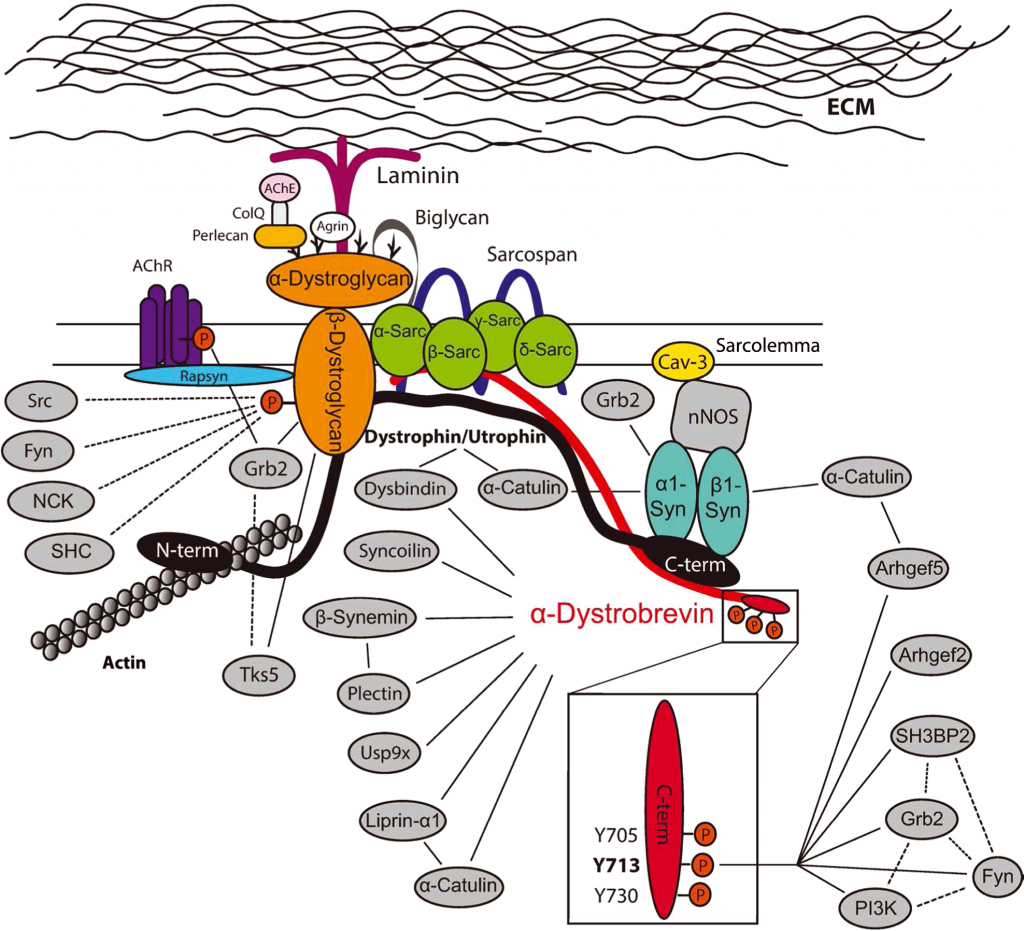
Gawor and Prószyński, NYAS 2018
A set of our projects is focused on the characterization of novel components of the Dystrophin-associated Glycoprotein Complex (DGC). This protein complex plays a pivotal role in the maintenance of muscle fiber integrity as well as in the organization and development of the postsynaptic machinery. On the molecular level, DGC helps to stabilize synaptic components by linking them to the actin cytoskeleton and the extracellular matrix. It also serves as a hub for the assembly of signaling molecules. We have recently identified novel synaptic proteins that are recruited to the DGC and now we perform more detailed studies on some of them.
Gawor and Prószyński, NYAS 2018
Pradhan BS and Proszynski TJ. “A Role for Caveolin-3 in the Pathogenesis of Muscular Dystrophies” Int. J. Mol. Sci. 2020, 21(22), 8736.
Bernadzki KM, Daszczuk P, Rojek KO, Pęziński M, Gawor M, Pradhan BS, de Cicco T, Bijata M, Bijata K, Włodarczyk J, Prószyński TJ, Niewiadomski P. “Arhgef5 Binds α-Dystrobrevin 1 and Regulates Neuromuscular Junction Integrity”. Front Mol Neurosci. 2020 Jun 10;13:104.
Bernadzki KM, Gawor M, Pęziński M, Mazurek P, Niewiadomski P, Rędowicz MJ, Prószyński TJ. “Liprin-α-1 is a novel component of the murine neuromuscular junction and is involved in the organization of the postsynaptic machinery”. Sci Rep. 2017 Aug 22;7(1):9116.
Gawor M and Prószyński TJ. “The molecular cross-talk of the dystrophin-glycoprotein complex”, concise review. Ann N Y Acad Sci. 2018 Jan;1412(1):62-72.
Gingras J, Gawor M, Bernadzki KM, Grady RM, Hallock P, Glass DJ, Sanes JR, Proszynski TJ. “Α-Dystrobrevin-1 recruits Grb2 and α-catulin to organize neurotransmitter receptors at the neuromuscular junction”. J Cell Sci. 2016 Mar 1;129(5):898-911.
Acetylcholine receptors (AChR)
Other projects aim in understanding how intracellular sorting of AChR is regulated. Despite decades of studies on AChR, little is known about the molecular machinery regulating AChR stability at the synapse as well as mechanisms of its intracellular transport.
Vaughan SK, Barbat-Artigas S, Rega K, Myers T, Proszynski TJ, Robitaille R, Valdez G. “Lynx1 modulates nAChRs in adult skeletal muscles and contributes to NMJ maintenance”. Under consideration, available as preprint at BioRxiv doi: https://doi.org/10.1101/2020.05.29.123851
Proszynski TJ and Sanes JR. „Amotl2 interacts with LL5b, localizes to podosomes and regulates postsynaptic differentiation in muscles”. J Cell Sci. 2013 May 15;126(Pt 10):2225-35.
Proszynski TJ, Gingras J, Valdez G, Krzewski K, Sanes JR. “Podosomes are present in a postsynaptic apparatus and participate in its maturation” PNAS. 2009 Oct 27;106(43):18373-8
Pradhan BS and Proszynski TJ. “A Role for Caveolin-3 in the Pathogenesis of Muscular Dystrophies” Int. J. Mol. Sci. 2020, 21(22), 8736.
Bernadzki KM, Daszczuk P, Rojek KO, Pęziński M, Gawor M, Pradhan BS, de Cicco T, Bijata M, Bijata K, Włodarczyk J, Prószyński TJ, Niewiadomski P. “Arhgef5 Binds α-Dystrobrevin 1 and Regulates Neuromuscular Junction Integrity”. Front Mol Neurosci. 2020 Jun 10;13:104.
Bernadzki KM, Gawor M, Pęziński M, Mazurek P, Niewiadomski P, Rędowicz MJ, Prószyński TJ. “Liprin-α-1 is a novel component of the murine neuromuscular junction and is involved in the organization of the postsynaptic machinery”. Sci Rep. 2017 Aug 22;7(1):9116.
Gawor M and Prószyński TJ. “The molecular cross-talk of the dystrophin-glycoprotein complex”, concise review. Ann N Y Acad Sci. 2018 Jan;1412(1):62-72.
Gingras J, Gawor M, Bernadzki KM, Grady RM, Hallock P, Glass DJ, Sanes JR, Proszynski TJ. “Α-Dystrobrevin-1 recruits Grb2 and α-catulin to organize neurotransmitter receptors at the neuromuscular junction”. J Cell Sci. 2016 Mar 1;129(5):898-911.
Commitment to the state-of-the-art:
We are strongly convinced that progress in science demands multidisciplinary approaches, collaborative efforts as well as development of new technologies facilitating innovative discoveries. Therefore, our group uses wide range of techniques and we often expand the repertoire for novel methods.
Molecular techniques:
We are strongly convinced that progress in science demands multidisciplinary approaches, collaborative efforts as well as development of new technologies facilitating innovative discoveries. Therefore, our group uses wide range of techniques and we often expand the repertoire for novel methods.
- DNA cloning
- RNA in vitro synthesis
- qRT-PCR and expression analysis
- Genotyping
Biochemistry:
- Western blotting
- Protein purification from bacterial and mammalian cells
- Co-immunoprecipitation and pull-down experiments
- Protein complex purification
- Mass spectrometry analysis
Cell culture:
- Culturing of various cell lines including primary neurons and muscle cells
- Immunocytochemistry
- Transfection of cells with DNA and mRNA
- Viral infection
Tissues:
- Animal surgery
- Dissection of muscles and brains
- Tissue electroporation
- Immunohistochemistry
- Live imaging of synapses in vivo
Conditional knockout mice and transgenics:
- Generation of conditional KO mice
- Mouse colony maintenance
- Genotyping
- Sperm freezing
- Behavioral studies
- Muscle physiology
- Pradhan BS, Prószyński TJ.
A Role for Caveolin-3 in the Pathogenesis of Muscular Dystrophies.
Int J Mol Sci. 2020 Nov 19;21(22):8736. 10.3390/ijms21228736. PMID: 33228026. DOI: 10.3390/ijms21228736 - Bernadzki KM, Daszczuk P, Rojek KO, Pęziński M, Gawor M, Pradhan BS, de Cicco T, Bijata M, Bijata K, Włodarczyk J, Prószyński TJ, Niewiadomski P.
Arhgef5 Binds α-Dystrobrevin 1 and Regulates Neuromuscular Junction Integrity.
Front Mol Neurosci. 2020 Jun 10;13:104. eCollection 2020. PMID: 32587503. DOI: 10.3389/fnmol.2020.00104 - Florkowska A, Meszka I, Zawada M, Legutko D, Proszynski TJ, Janczyk-Ilach K, Streminska W, Ciemerych MA, Grabowska I.
Pax7 as molecular switch regulating early and advanced stages of myogenic mouse ESC differentiation in teratomas.
Stem Cell Res Ther. 2020 Jun 17;11(1):238. DOI: 10.1186/s13287-020-01742-3 - Pęziński M, Daszczuk P, Pradhan BS, Lochmüller H, Prószyński TJ.
An improved method for culturing myotubes on laminins for the robust clustering of postsynaptic machinery.
Sci Rep. 2020 Mar 11;10(1):4524. DOI: 10.1038/s41598-020-61347-x - Alvarez-Suarez P, Gawor M, Prószyński TJ.
Perisynaptic schwann cells – The multitasking cells at the developing neuromuscular junctions.
Semin Cell Dev Biol. 2020 Aug;104:31-38. DOI: 10.1016/j.semcdb.2020.02.011 - Rojek KO, Krzemień J, Doleżyczek H, Boguszewski PM, Kaczmarek L, Konopka W, Rylski M, Jaworski J, Holmgren L, Prószyński TJ.
Amot and Yap1 regulate neuronal dendritic tree complexity and locomotor coordination in mice.
PLoS Biol. 2019 May 1;17(5):e3000253. DOI: 10.1371/journal.pbio.3000253 - Kepser LJ, Damar F, De Cicco T, Chaponnier C, Prószyński TJ, Pagenstecher A, Rust MB.
CAP2 deficiency delays myofibril actin cytoskeleton differentiation and disturbs skeletal muscle architecture and function.
Proc Natl Acad Sci U S A. 2019 Apr 23;116(17):8397-8402. DOI: 10.1073/pnas.1813351116 - Pigna E, Simonazzi E, Sanna K, Bernadzki KM, Proszynski T, Heil C, Palacios D, Adamo S, Moresi V.
Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis.
EBioMedicine. 2019 Feb;40:717-732. DOI: 10.1016/j.ebiom.2019.01.038 - Gawor M, Prószyński TJ.
The molecular cross talk of the dystrophin-glycoprotein complex.
Ann N Y Acad Sci. 2018 Jan;1412(1):62-72. DOI: 10.1111/nyas.13500 - Bernadzki KM, Gawor M, Pęziński M, Mazurek P, Niewiadomski P, Rędowicz MJ, Prószyński TJ.
Liprin-α-1 is a novel component of the murine neuromuscular junction and is involved in the organization of the postsynaptic machinery.
Sci Rep. 2017 Aug 22;7(1):9116. DOI: 10.1038/s41598-017-09590-7 - Waschek JA, Cohen JR, Chi GC, Proszynski TJ, Niewiadomski P.
PACAP Promotes Matrix-Driven Adhesion of Cultured Adult Murine Neural Progenitors.
ASN Neuro. 2017 May-Jun;9(3):1759091417708720. DOI: 10.1177/1759091417708720 - Gingras J, Gawor M, Bernadzki KM, Grady RM, Hallock P, Glass DJ, Sanes JR, Proszynski TJ.
Α-Dystrobrevin-1 recruits Grb2 and α-catulin to organize neurotransmitter receptors at the neuromuscular junction.
J Cell Sci. 2016 Mar 1;129(5):898-911. DOI: 10.1242/jcs.181180 - Karolczak J, Pavlyk I, Majewski Ł, Sobczak M, Niewiadomski P, Rzhepetskyy Y, Sikorska A, Nowak N, Pomorski P, Prószyński T, Ehler E, Rędowicz MJ.
Involvement of unconventional myosin VI in myoblast function and myotube formation.
Histochem Cell Biol. 2015 Jul;144(1):21-38. DOI: 10.1007/s00418-015-1322-6 - Bernadzki KM, Rojek KO, Prószyński TJ.
Podosomes in muscle cells and their role in the remodeling of neuromuscular postsynaptic machinery.
Eur J Cell Biol. 2014 Oct;93(10-12):478-85. DOI: 10.1016/j.ejcb.2014.06.002 - Proszynski TJ, Sanes JR.
Amotl2 interacts with LL5β, localizes to podosomes and regulates postsynaptic differentiation in muscle.
J Cell Sci. 2013 May 15;126(Pt 10):2225-35. DOI: 10.1242/jcs.121327 - Oh HJ, Abraham LS, van Hengel J, Stove C, Proszynski TJ, Gevaert K, DiMario JX, Sanes JR, van Roy F, Kim H.
Interaction of α-catulin with dystrobrevin contributes to integrity of dystrophin complex in muscle.
J Biol Chem. 2012 Jun 22;287(26):21717-28. DOI: 10.1074/jbc.M112.369496 - Proszynski TJ, Gingras J, Valdez G, Krzewski K, Sanes JR.
Podosomes are present in a postsynaptic apparatus and participate in its maturation.
Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18373-8. DOI: 10.1073/pnas.0910391106 - Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K.
Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network.
J Cell Biol. 2009 May 18;185(4):601-12. DOI: 10.1083/jcb.200901145 - Balwierz A, Czech U, Polus A, Filipkowski RK, Mioduszewska B, Proszynski T, Kolodziejczyk P, Skrzeczynska-Moncznik J, Dudek W, Kaczmarek L, Kulig J, Pryjma J, Dembinska-Kiec A.
Human adipose tissue stromal vascular fraction cells differentiate depending on distinct types of media.
Cell Prolif. 2008 Jun;41(3):441-59. DOI: 10.1111/j.1365-2184.2008.00531.x - Proszynski TJ, Klemm R, Bagnat M, Gaus K, Simons K.
Plasma membrane polarization during mating in yeast cells.
J Cell Biol. 2006 Jun 19;173(6):861-6. DOI: 10.1083/jcb.200602007 - Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, Wagner J, Kozak K, Grabner H, Walzer K, Bagnat M, Simons K, Walch-Solimena C.
A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast.
Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):17981-6. DOI: 10.1073/pnas.0509107102 - Konopka W, Duniec K, Mioduszewska B, Proszynski T, Jaworski J, Kaczmarek L.
hCMV and Tet promoters for inducible gene expression in rat neurons in vitro and in vivo.
Neurobiol Dis. 2005 Jun-Jul;19(1-2):283-92. DOI: 10.1091/mbc.e03-07-0511 - Proszynski TJ, Simons K, Bagnat M.
O-glycosylation as a sorting determinant for cell surface delivery in yeast.
Mol Biol Cell. 2004 Apr;15(4):1533-43. DOI: 10.1091/mbc.e03-07-0511 - Jaworski J, Mioduszewska B, Sánchez-Capelo A, Figiel I, Habas A, Gozdz A, Proszynski T, Hetman M, Mallet J, Kaczmarek L.
Inducible cAMP early repressor, an endogenous antagonist of cAMP responsive element-binding protein, evokes neuronal apoptosis in vitro.
J Neurosci. 2003 Jun 1;23(11):4519-26. DOI: 10.1523/JNEUROSCI.23-11-04519.2003 - Jaworski J, Figiel I, Proszynski T, Kaczmarek L.
Efficient expression of tetracycline-responsive gene after transfection of dentate gyrus neurons in vitro.
J Neurosci Res. 2000 Jun 15;60(6):754-60. DOI: 10.1002/1097-4547(20000615)60:6<754::AID-JNR7>3.0.CO;2-M

